This amazing news is 8 years in the making!
CONGRATULATIONS to all of the eye care surgeons, practitioners and researchers in the field who have been involved in the ongoing effort to get Keratoconus patients a safe and effective option for the corneal disease they have to battle!
On Monday, April 18th, a version of Corneal Crosslinking aka “CXL” was FINALLY approved by the FDA!
We are pleased to announce this as these efforts were started 8 long years ago with our Doctors and Center as a part of it!
In 2008, our Center was selected as 1 of the 12 sites that participated in the FDA clinical trials to gain approval for this procedure.
The specific version of CXL that was approved yesterday is the Epithelial-Off or “Epi-off” version of Crosslinking for keratoconus patients.
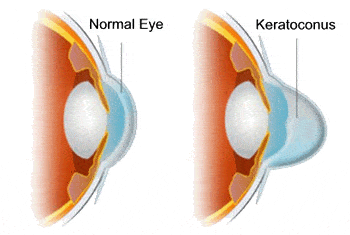
We hope that this approval does several things, the MOST important being that more doctors identify this disease at an earlier age, since now there is an FDA-approved treatment available.
Also, we hope that in 1-2 years, insurance coverage will be more routine and will cover it. That is a current challenge with this procedure.
“This FDA approval has been highly anticipated by the keratoconus community,” said Mary Prudden, Executive Director for the National Keratoconus Foundation. “Corneal cross-linking provides patients a much-needed option to treat this debilitating disease. Patients suffering from progressive keratoconus can now receive a therapeutic treatment that has been rigorously tested and approved.”
Our Doctors at the Center for Excellence in Eye Care have helped thousands of patients since we started working with CXL eight years ago and for that we are so grateful to have been a part of this effort! Congratulations to all!
Please see below for a link to the press release about the approval and call us today if you or a loved one has been diagnosed with Keratoconus or Ectasia! 305-598-2020
For more info on finding a doctor to treat Keratoconus in your area, or to see the doctors at our center who perform CXL, please click here.

